[ Breaking News! ] First MASH treatment approved by FDA
On March 14th, industry-altering news was published.
Do you know already what we are talking about?
The United States Food and Drug Administration (FDA) announced their approval of Rezdiffra (resmetirom), developed by Madrigal Pharmaceuticals, as the first treatment for MASH.
FDA approved first MASH treatment
We are truly excited that a drug has been approved for a disease for which there had previously been no treatment.
With this in mind, if you are also developing a MASH drug, our STAMTM-HCC/IO+ model allows you to most effectively evaluate your compound’s efficacy.
Although Rezdiffra has been approved as a treatment for MASH,
clinical trial results indicate that at least half of MASH patients did not show any improvement in their condition.
This is thought to be due to the complexity of the mechanism of the disease MASH, and
is one of the reasons why therapeutic drug development for MASH has not been successful to date.
Our STAMTM-HCC/IO+ model reflects the complex pathology that occurs in MASH.
Compounds for various pathways have been evaluated in our model, and many progressed to clinical trials afterwards.
The figure below shows compounds that have undergone Phase 2 clinical trials or
further, as well as their targets.
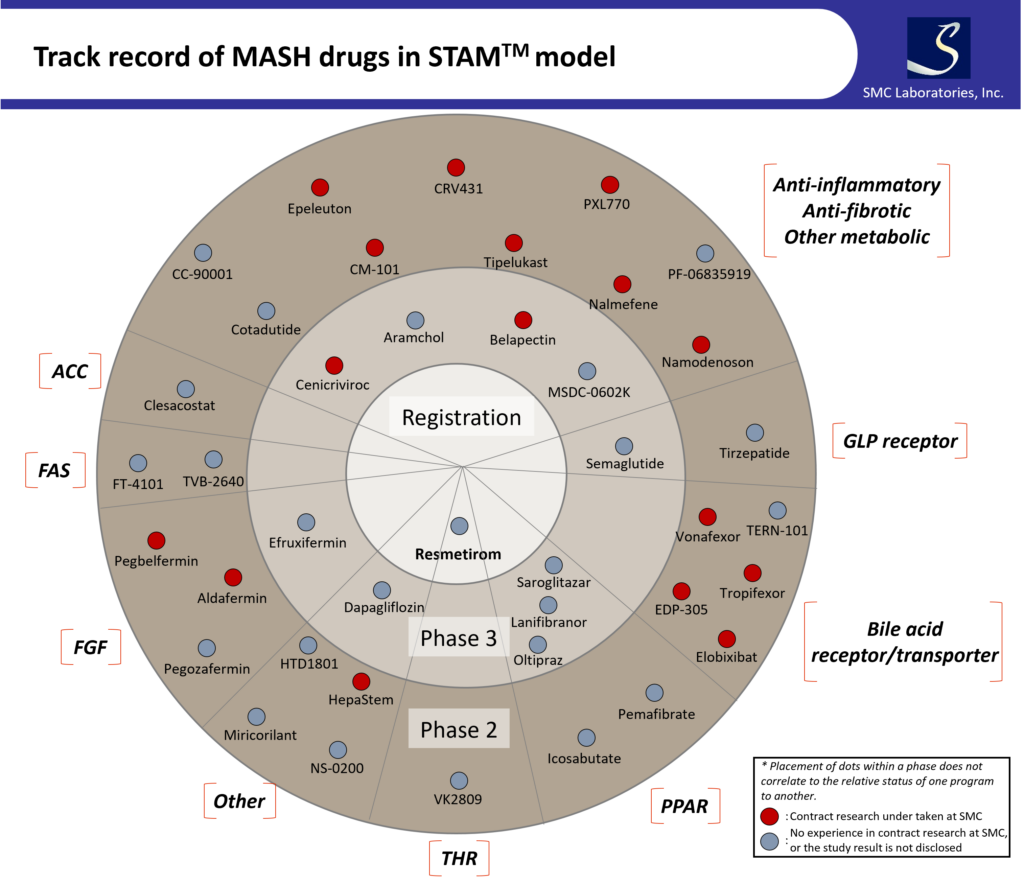
As you can see in the diagram above, the STAMTM-HCC/IO+ model reproduces the
same complex pathology seen in MASH and is capable of evaluating various targets.
This model, which reproduces the complexity of MASH, allows you to evaluate your compounds as monotherapy, or as a combined administration with Rezdiffra. We have evaluated combined compound administration in the past and have observed synergistic effects.
With our expertise and experience conducting more than 800 drug efficacy evaluation studies using our STAMTM-HCC/IO+ model, we are confident that we can propose optimal suggestions to match your needs.
We believe that this historical news will be the first step in the development of future research. We would be delighted if the results of the approval could help us supporting your own research. Please take this opportunity to contact us.
