SERVICES
Pharmacology study & consulting service
We support cutting-edge research by receiving requests for pharmacology study from over 1,000 pharmaceutical companies and bio-tech in 30 countries around the world.
Service overview
Pharmacology study
Providing drug efficacy evaluation study using inflammation and fibrosis model mice
Histopathological analysis (paraffin block preparation, staining, and scoring)
- Providing analysis results of clinical specimens from rodents
Specialized area
Pathological model mice with inflammation and fibrosis as keywords
Well-versed with fibrosis models for each organ (liver, skin, kidney, lung, intestine)
Histopathological analysis
- Establishing a new immunostaining assay and scoring method using our accumulated knowledge and technique from evaluating MASH/NASH and fibrosis
- In MASH/NASH/fibrosis models, we can identify balloon-like degeneration in our client produced specimens, which is known to be difficult to evaluate by a third party in Non-clinical pharmacology studies
Non-clinical pharmacology studies
- High-quality drug efficacy and pharmacology testing based on our research expertise
- Comprehensive Line-up of various disease models related to fibrosis, inflammation, metabolic disorders, and cancer
- Strategic research design proposal from our experienced scientists
Benefits for our clients
- Objective data package
- Established pharmacological studies
We are a non-clinical CRO with extensive experience in various disease area based in Tokyo.
We own many established disease models including ”fibrosis”.
Our animal facilities are approved by the National Institutes of Health (NIH). We have obtained Animal Welfare Assurance approval and you can receive our contracted services utilizing subsidies from the U.S. Public Health Service (PHS).
Histological imaging
- High-quality analysis, Proven skills in histology
- Detailed and comprehensive report by our experts
- Professional support for data publication/IND applications
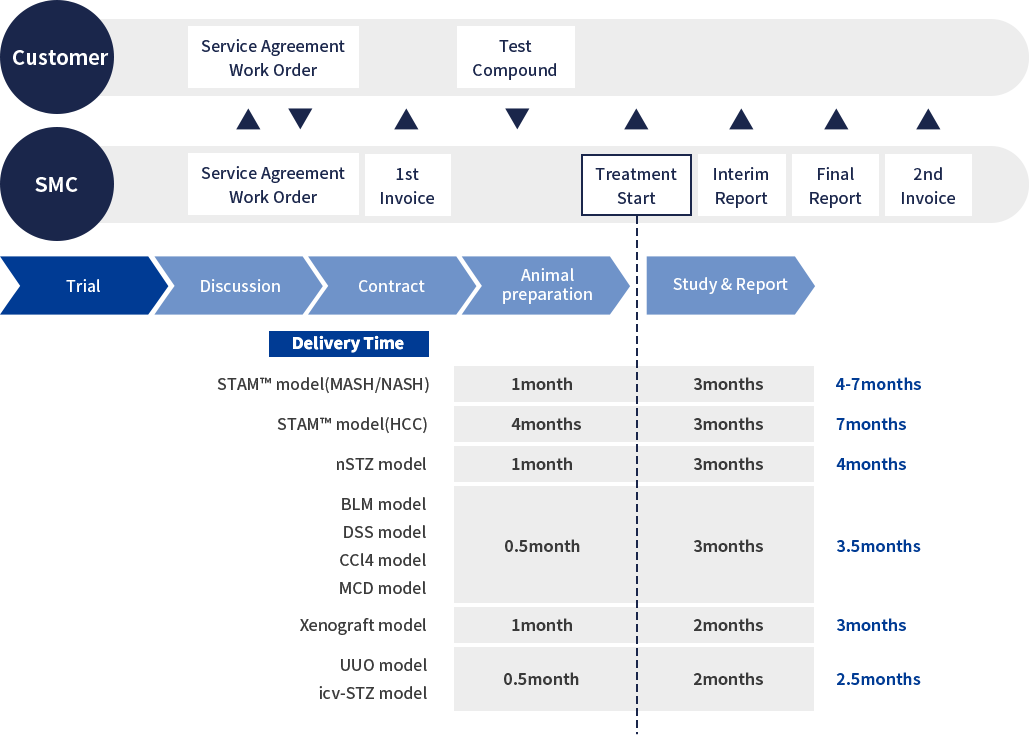
Our CRO service process
- At SMC, we work closely with the client to fully discuss the study design and content so that it can be tailored to the clients needs.
- A non-disclosure agreement (NDA) is available if necessary.
- Once a study design has been agreed upon, we prepare a study protocol and discuss the fine details of the study content with the client.
- At this point, we also prepare a Master Services Agreement (MSA) in order to proceed smoothly with contractual procedures.
- When all the details of the study protocol have been finalized, we provide the client with a formal quote and work order form.
- After receiving the signed work order, the first invoice will be issued, and study preparation will begin.
- The client needs only to make preparations for the shipping of the test substance, and SMC will carry out the rest.
- During the treatment period, we keep in close contact with the client, providing updates on the general condition and weight changes of the mice every week.
- At the end of the in-life portion of the study, samples are collected and analysis is performed according to the study protocol.
- Analysis results can be submitted as interim data before the final report is delivered, and we are able to be flexible with deadline scheduling should there be a specific date by which data is required.
- After analysis is complete, a study report is compiled and submitted. The second invoice is issued with the report submission.
- We welcome discussion of the study results between clients and our research team, and are happy to share our interpretation of the results and suggestions going forward.
Histology
Our pathology specialists support the construction of experimental analysis systems, data acquisition and explanation of results, and maximize the information from the histology samples of pharmacology studies conducted by clients.
ABOUT HISTOLOGY
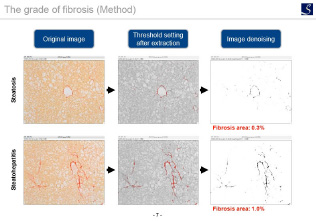
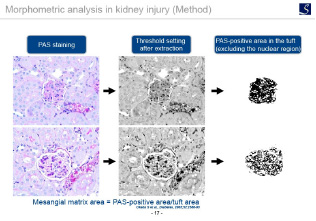
With our expertise in inflammation and fibrosis, we will perform histopathological quantification and scoring for each tissue.
List of evaluation items
-
Fibrosis evaluation
alpha-SMA immunostaining, ER-TR7 immunostaining, Sirius red staining, Masson trichrome staining, etc.
-
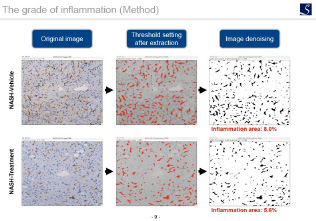
Inflammation evaluation
F4 / 80 immunostaining, Gr-1 immunostaining, CD4 immunostaining, etc.
-
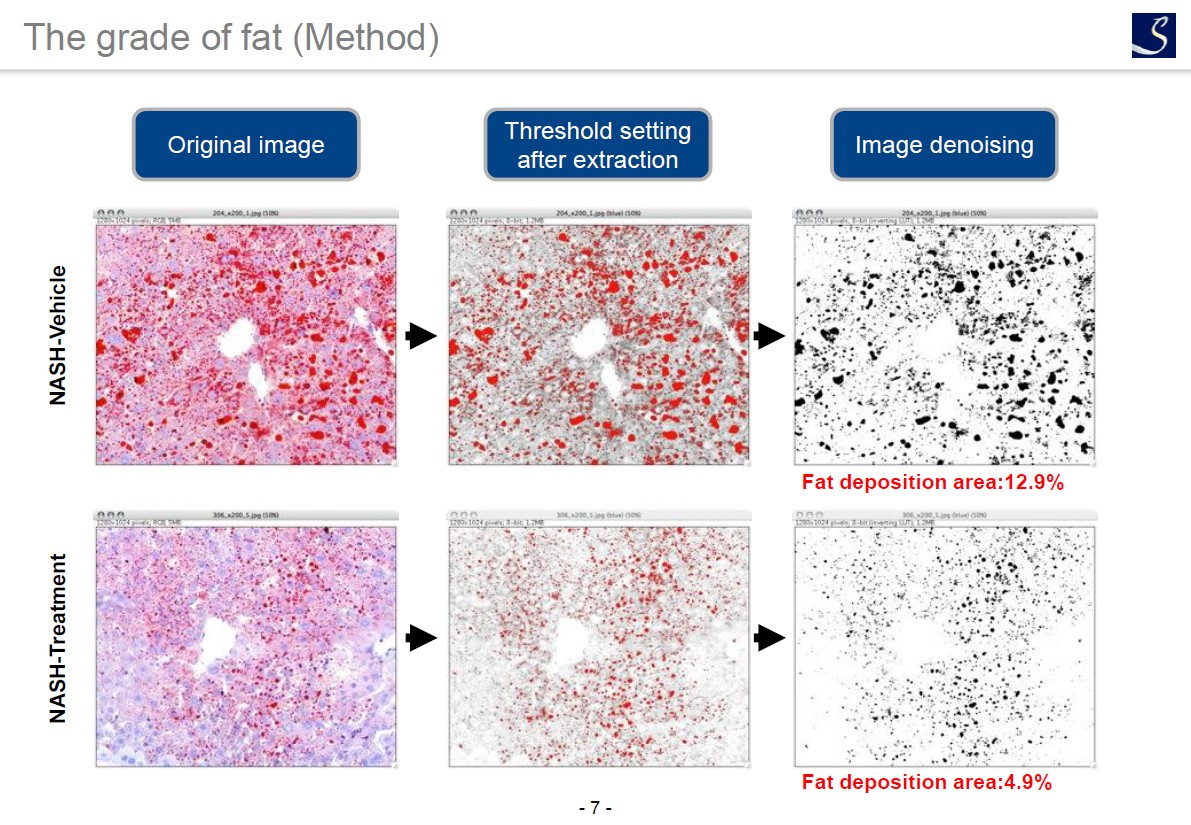
Fat deposition evaluation
Oil red staining
-
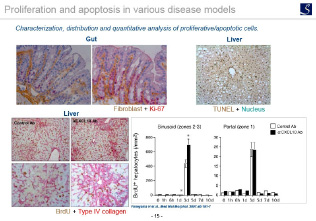
Cell proliferation / apoptosis evaluation
Ki67 immunostaining, TUNEL staining, etc.
-
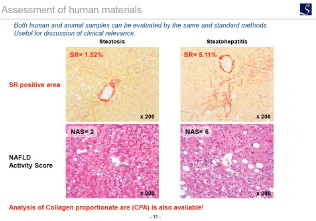
Human tissue evaluation
HE staining, Sirius red staining, etc.
Utilizing our expertise in fibrosis, we provide a fibrosis analysis method suitable for each disease model tissue.
-
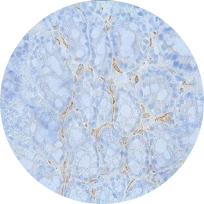
Alpha-SMA immunostaining
Model: DSS model
-
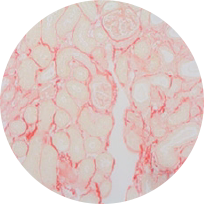
Sirius red staining
Model: UUO model
-
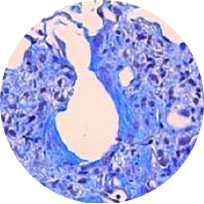
Masson trichrome staining
Model: BLM model
SUMMARY OF SERVICE
Processing & Embedding
-
Embedding of human and animal tissues in paraffin (FFPE blocks) from fixed tissues.
- Embedding of human and animal tissues in OCT (frozen OCT embedded tissues) from fixed tissues.
Instruction of fresh frozen block preparation for frozen sections.
- Cutting of sections (4-8 µm thick) from paraffin and O.C.T. embedded blocks.
- Preparation of serial thin sections.
Routine staining
- HE, Sirius red, Masson trichrome, PAS, Oil red, etc.
Immunohistochemistry (IHC): Inflammation-related molecules, fibrosis-related molecules etc.
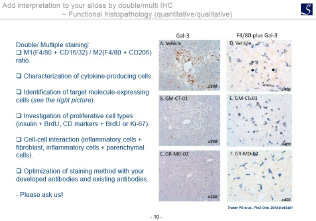
- Functional Immunohistology(Double/multiple staining).
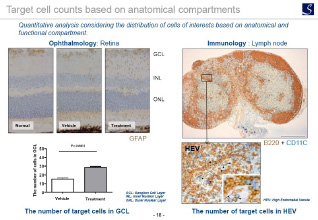
Imaging analysis
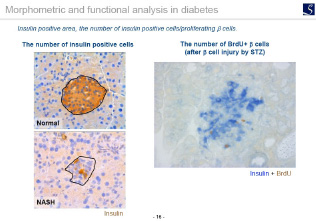
- Area, length, diameter, stained cell count, percentage positive area, shape etc.
- Proliferation, Apoptosis, pathological grading etc.
Interpretation of results
- Discussion of the histological findings in view of pharmacology.
User support
- We can also set up a place for us to explain our findings and have a discussion with our researchers.
HISTOPATHOLOGY AND IMAGING SERVICES FOR MASH(f.k.a. NASH,hereinafter referred as MASH/NASH)
Histopathological parameters are important endpoints in nonclinical as well as clinical studies in NASH and liver fibrosis.
SMC’s outstanding performance in MASH/NASH/liver fibrosis research
-
As a leading CRO in MASH/NASH, SMC has accumulated know-how by assessing over 500 pharmacology studies and 50,000 slides of MASH/NASH and related diseases in mouse, rat and human.
- judgment of NAS (especially, ballooning), assessment of pathological changes from disease control, discussion of clinical relevance… and more!NAS; NAFLD Activity score
For…
- Assessment the grade of disease (MASH/NASH, fibrosis) in not only nonclinical pharmacology studies but also clinical studies.
- Development of animal models (including Tg and KO mice) for drug evaluation in the clients’ own laboratory.
- Achievement of high quality report/data package with objective evaluation.
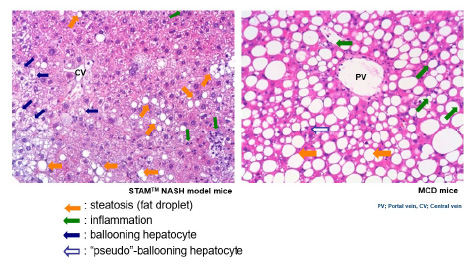
NAS: "BALLOONING" OR "PSEUDO-BALLOONING"
NAS (including ballooning, inflammation, steatosis) is an important parameter to evaluate the drug efficacy in MASH/NASH in both human and animal models.
In order to translate nonclinical results into clinical practice, it is essential to discriminate “true”- from “pseudo”-ballooning in disease models.
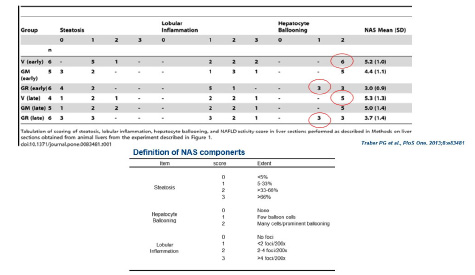
THE GRADE OF NAS: ACHIEVEMENT
An accurate scoring can evaluate the efficacy of the test substances on each components (steatosis, lobular inflammation, hepatocyte ballooning) of NAS.
HISTOLOGY ASSESMENT METHOD & MATERIALS
HISTOLOGY ASSESMENT DISEASE MODELS
Trial
Centered around our proprietary STAM™ model, we offer a lineup of 25 highly-rated SMC quality models. We also provide transportation, material supply, and other services in order meet your needs.
Disease model mice / Biological sample service
On top of supplying pathological model mice, SMC Laboratories can also provide clients with blood, tissue, and other various biological samples. The approximate delivery date for samples from each model mouse varies.
Target organ
- Whole blood
- Plasma
- Serum
- Brain
- Eyeball
- Heart
- Stomach
- Lung
- Liver
- Kidney
- Gastrocnemius/
soleus muscle - Skin from the back
- Thigh muscle
- Thymus
- Genitals
- Small intestine
- Large intestine
- Caecum
- White adipose tissue
- Brown adipose tissue
Models
From the preparation of our disease models, to the pharmacological analysis and pathological evaluation, we consider everything in regards to the clinical research process.
In other words, we strive to make our research easily translatable to the clinical research phase.
Model preparation: Our models have clear similarities with clinical onset mechanisms
Pathology: Our models utilize the same pathological evaluation methods as clinical trials