MODEL LINEUP
STAM™ Model | MASH & Fibrosis & HCC
What can the STAM™ model be used for?
The STAM™ model is SMC Laboratories’ proprietary mouse model for pharmacological drug evaluation of Steatohepatitis, MASH, Liver Fibrosis and Hepatocellular carcinoma (HCC). Below, you will find information such as about the model creation and key endpoints for evaluation of drug candidates against MASLD / MASH and Liver Fibrosis. For more information about how to utilize this model as liver cancer model, please also check the page “STAM HCC/IO+”.
How is the STAM™ model created?
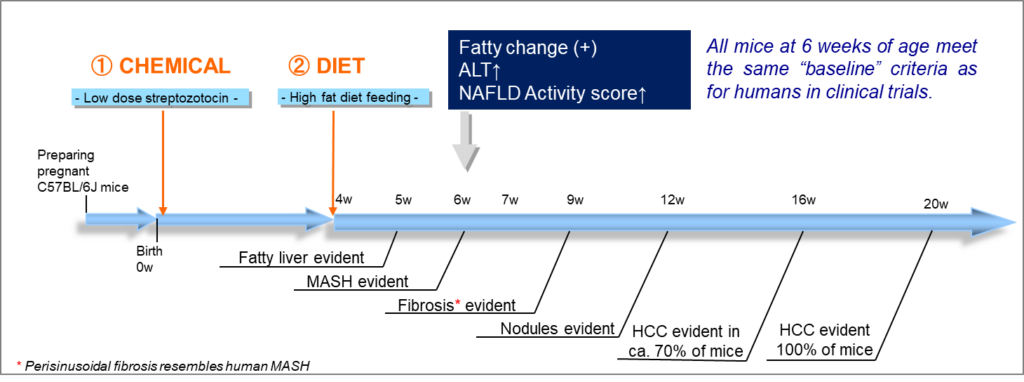
Fig. 1: STAM™ model creation and disease progression from fatty liver disease to HCC (w: weeks of age)
In this model, male C57BL/6 mice aged two days are given a single subcutaneous dose of streptozotocin to reduce insulin secretory capacity. When the mice turn four weeks old, we start feeding them a high-fat diet. Since this model has a background of late-stage type 2 diabetes mellitus, the high-fat diet will induce the development of Metabolic Dysfunction-Associated Steatotic Liver Disease. From there, the mice progress to MASH, liver fibrosis and at a later timepoint show spontaneous HCC development.
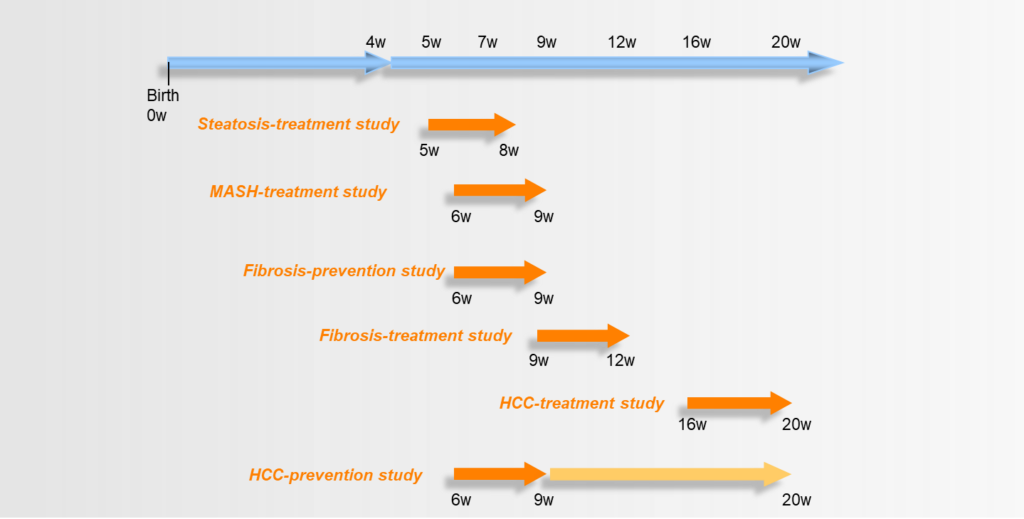
Fig. 2: Different treatment periods for various pharmacological drug efficacy studies. For example, the suggested treatment period for targeting MASH in this model is from 6 to 9 weeks of age. (w: weeks of age)
Compared to other MASH model mice, the disease progresses in a relatively short period of time of only a few weeks. Also, it follows a reliable stepwise progression, which facilitates the decision for the correct treatment period. Also, when compared to other diet-induced HCC models, the mice show a quick disease onset with a reliable 100% progression to HCC by 20 weeks of age.
Analysis Items & Key endpoints
We offer a rather large number of different analyses to evaluate the efficacy of your drug candidate. Below, we only list the main analysis items and key endpoints included in the standard package for this model.
For MASH / Fibrosis studies
HE staining to evaluate the NAFLD activity score (NAS) (including steatosis, lobular inflammation and hepatocyte ballooning scores)
Oil Red staining for evaluation of fat deposition
Sirius Red staining to evaluate severity of liver fibrosis
Plasma ALT & AST as liver injury markers
Plasma & liver triglycerides
Blood glucose levels
Body & liver weight
For HCC studies
Tumor growth rate evaluation by microCT (evaluation of growth inhibition)
Macroscopic evaluation of visible tumor number and size
Survival rate
Body weight & Liver weight
Optional: Immunohistochemistry staining (IHC)
If you would like to get more information on our capabilities regarding any specific analysis item (even when the item is not listed above), please use the contact button at the bottom of the page and we will be happy to provide you with additional information.
Comparison between STAM™ Model and other MASH mouse models
When comparing the different MASH mouse models, it becomes clear that the STAM™ model certainly has a lot of benefits. Those can be used for the evaluation of drug candidates against MASLD/MASH as well as MASH-derived liver fibrosis. The model shows most of the diagnostic parameters of human MASH and can therefore be used as stand-alone model or in a model-combination approach.
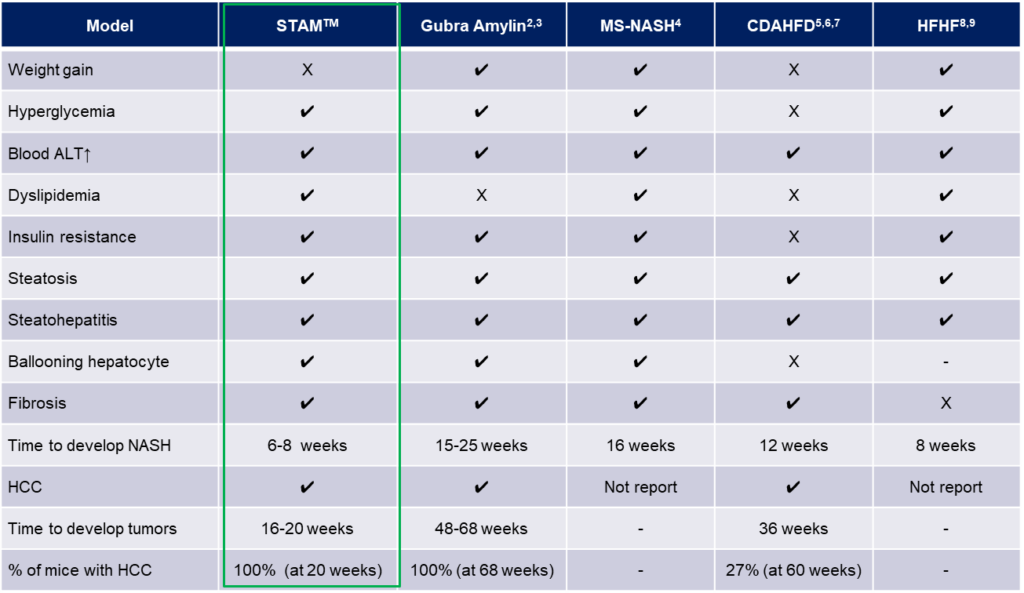
Fig. 3: Comparison table of STAM™ Model with other MASH model mice
While there is no MASH model on the market that perfectly mimics the human pathology 1-to-1, the STAM™ model provides a solid base for your drug evaluation and show most of the critical parameters that define MASH disease. A very common approach is the drug evaluation in a combination of different models to confirm its pharmacological efficacy and smoothen the path to the next stage of IND filing.
The STAM™ Model in Immuno-Oncology (IO)
SMC Laboratories has a proven track record of evaluating a number of different immune checkpoint inhibitors (ICI) such as PD-1 or PD-L1 in the STAM™ HCC model. We could demonstrate across various studies that the treatment with ICIs lead to an increase of intra-tumoral and peri-tumoral CD8 positive cells, as well as a decrease in the growths of tumor nodules.
For detailed information about the STAM™ HCC/IO+ model as liver cancer model and our expertise regarding Immuno-Oncology studies, please have a look at the page “STAM™ HCC/IO+”.
Contact us
If you have any questions about our STAM™ model or if any of the following points applies to you, please feel free to contact us through the contact button below.
I would like a more in-depth explanation about this model.
I would like to discuss our project with SMC’s science team.
I need support to choose the best-fitting model for our project.
I have some other concerns or open questions that I would like to discuss.
Please reach out to us. We are happy to assist you!
