SMC MODELS
CDAHFD Model
The CDAHFD model
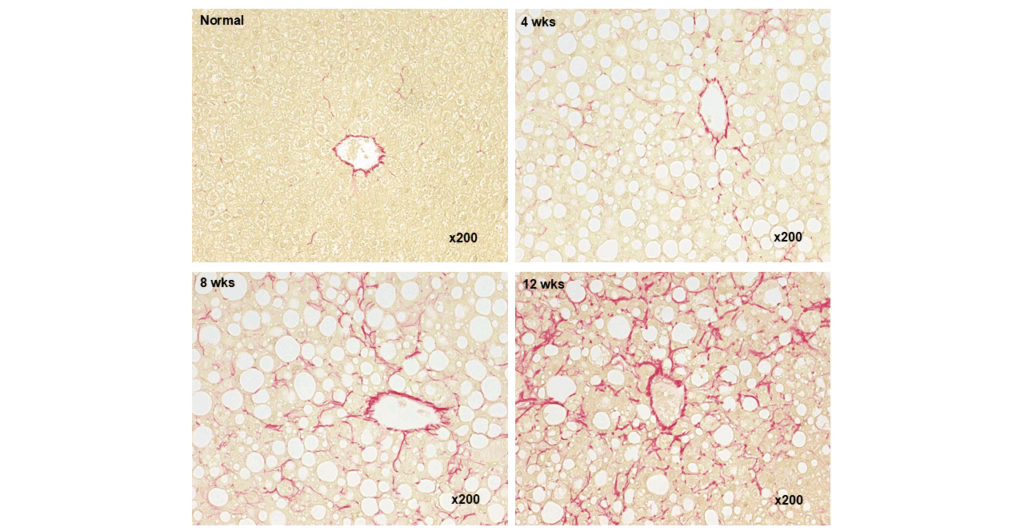
The CDAHFD model is a widely used and well-characterized model for the evaluation of pharmacological efficacy of new treatments against liver inflammation, liver fibrosis and fatty liver diseases such as MASLD (formerly: NAFLD). The model shows a strong development of steatosis and inflammation with a significant increase of fibrotic area in the liver.
How we create the CDAHFD model
The CDAHFD model is induced by choline-deficient, L-amino acid-defined, high fat diet and develops the typical pathology within just 12 weeks of this dietary intervention. The model is based on 6-weeks old, male C57BL/6J mice.
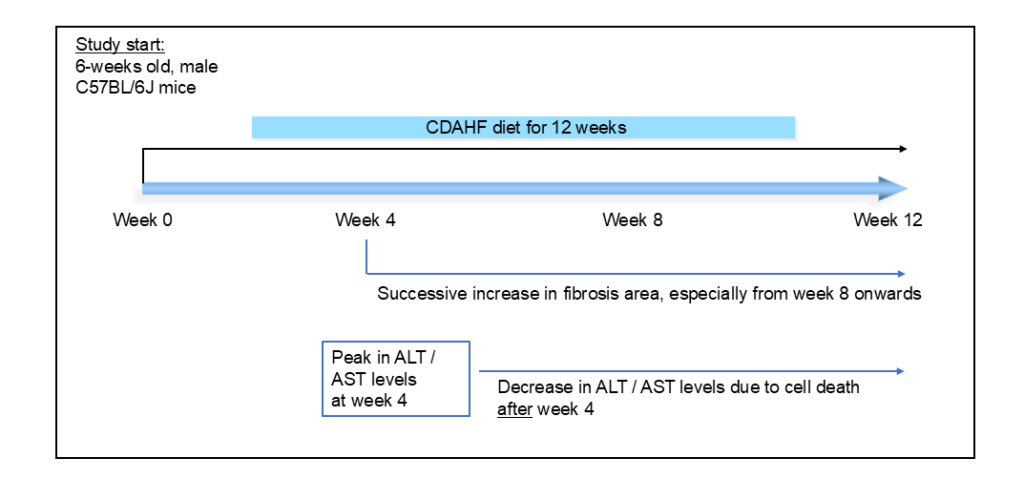
Analysis items and key endpoints
Histopathological analysis
HE staining (Inflammation score, Steatosis score)
Sirius Red staining (Fibrosis area)
Biochemistry analysis
ALT / AST (liver injury markers)
Gene expression analysis
TNFa
NLRP3
etc.
Other parameters
Body weight
Liver weight
Body-liver weight ratio
Liraglutide as Positive Control
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist often used as once-daily injection to treat human patients with type 2 diabetes and for chronic weight management. It acts on the pancreas stimulating insulin release, when blood sugar levels are high and suppressing glucagon secretion, a hormone that increases glucose production by the liver. Furthermore, it slows down gastric emptying (digestion), which helps control blood sugar spikes after meals and reduces appetite by acting on receptors in the brain that regulate hunger.
However, in SMC Laboratories’ CDAHFD mouse model the treatment with Liraglutide from week 6 to 12 predominantly demonstrated efficacy for significantly reducing the fibrosis positive area.
SMC Laboratories offers a number of different mouse models for liver diseases such as our proprietary STAM model for the evaluation of treatments against MASH, fibrosis or HCC. Get more information about the STAM model.
