Addressing Unmet Needs with Tailored Non-Clinical Solutions
Idiopathic Pulmonary Fibrosis (IPF) remains a high-priority therapeutic area, where current standard-of-care (SOC) treatments such as Nintedanib and Pirfenidone help slow disease progression, but do not halt it entirely. Despite treatment, mortality remains high, and there is limited improvement in quality of life and overall survival. The urgent need for safer and more effective therapies continues.
Why Drug Development in IPF Doesn’t Stop – Even With SOC
Limitations of current treatments:
– Delay FVC decline but do not fully halt progression
– Limited survival benefit
– Associated with adverse effects (e.g., GI symptoms, photosensitivity)
To overcome these limitations, next-generation therapies must demonstrate both enhanced efficacy and improved safety.
The Rising Bar in Clinical Trials: Nonclinical Studies Must Evolve Too
Clinical development of new IPF drugs increasingly requires:
– Add-on trials comparing new agents in combination with SOC
– Monotherapy trials versus placebo in SOC-naïve patients
– Evidence beyond FVC: acute exacerbation prevention, QOL improvements, etc.
Meeting these evolving requirements hinges on the ability to generate high-quality, translatable nonclinical data early in development.
What Matters Now in IPF Drug Discovery
To enhance your candidate’s value and differentiation, nonclinical studies are expected to address:
– Stronger anti-fibrotic activity
– Symptom relief (e.g., cough, dyspnea) and improved quality of life
– Prevention or treatment of acute exacerbations
– Combination therapy strategies
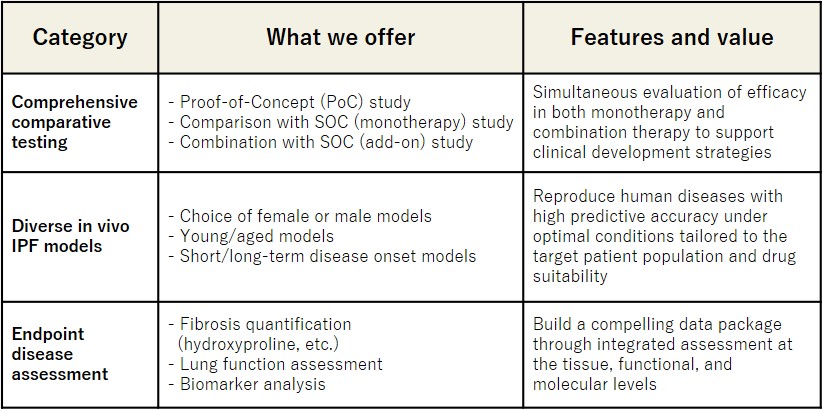
SMC Laboratories’ IPF Solutions