A New Phase in MASH Therapeutics: Resmetirom (THR-β Agonist) and Semaglutide (GLP-1 Receptor Agonist)
In 2024, the U.S. FDA approved Resmetirom (a THR-β agonist) as the first therapeutic agent for MASH. In August 2025, Semaglutide (a GLP-1 receptor agonist) was approved as the second therapeutic agent for this indication.
The two agents differ in their mechanisms of action: Resmetirom is a THR-β agonist, while Semaglutide is a GLP-1 receptor agonist.
The table below summarizes the clinical trial data of Resmetirom (MAESTRO-NASH) and Semaglutide (ESSENCE).
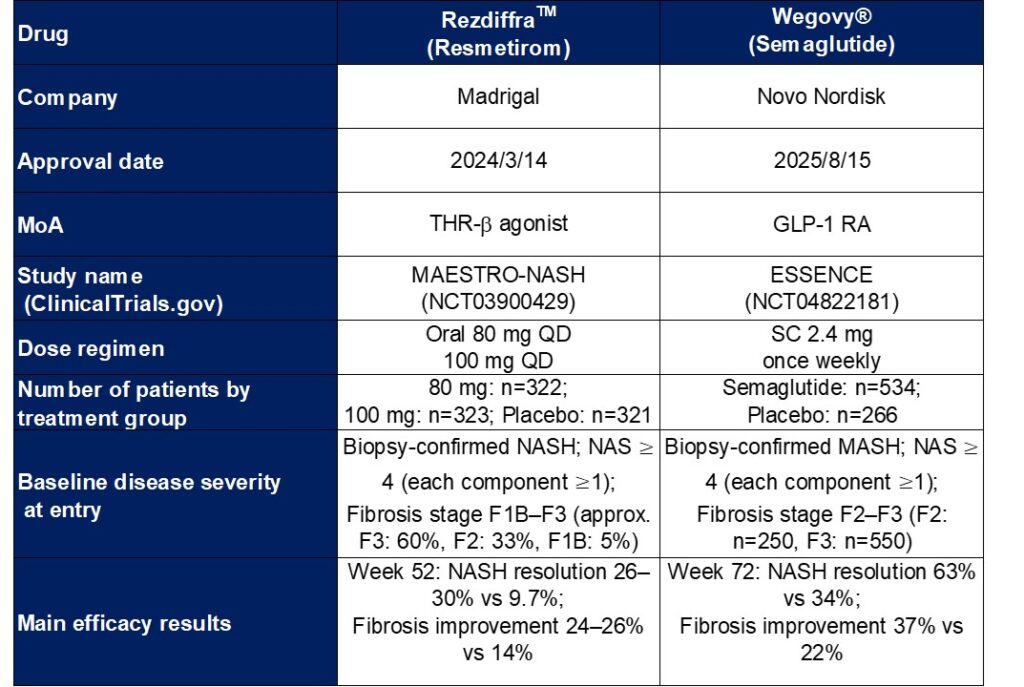
Resmetirom (THR-β agonist) directly improves hepatic lipid metabolism, thereby achieving balanced improvements in MASH resolution and fibrosis reduction. Semaglutide (GLP-1 receptor agonist) improves systemic metabolism, leading to a higher rate of MASH resolution as well as significant fibrosis improvement.
At SMC, we have evaluated both compounds in the STAM™ mouse model and confirmed improvements in NAS and fibrosis. These results are consistent with clinical trial outcomes, demonstrating that clinically relevant endpoints can be addressed at the preclinical stage.
Through these endpoints, SMC provides preclinical evaluation services that support the development of MASH therapeutics, including Resmetirom and Semaglutide.
For further details, please feel free to contact us.
