MODEL LINEUP
Porcine Pancreatic Elastase (PPE) model
The PPE-induced COPD model
The pathology of chronic obstructed pulmonary disease (COPD) in mice can be induced using different methods such as the smoke-induced model and the Elastase-induced emphysema model. Each of the models has its’ advantages and disadvantages that allow for the evaluation of new drug candidates. In the following, you will learn about the PPE-induced COPD model that we use at SMC Laboratories for pharmacological efficacy studies.
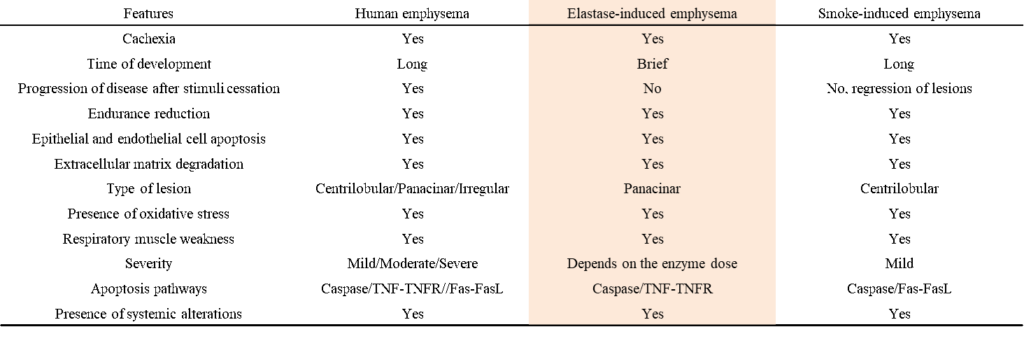
Fig. 1: Comparison table of human COPD to 2 different COPD-animal models. (An Acad Bras Cienc 2011;83:4)
Some of the benefits in utilizing the porcine pancreatic elastase (PPE)-induced emphysema model is that it shows a rapid onset of the disease and provides easy to measure functional changes in the mice’ lungs. Furthermore, the lesion severity is directly correlated to the enzyme dose and therefore allows for simple adjustment to your preclinical needs.
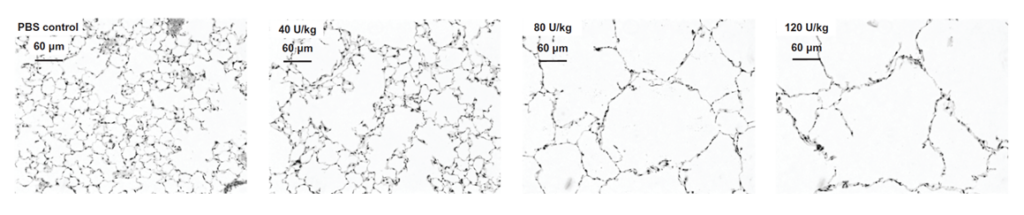
Fig. 2: Dose dependent lesion severity in the lungs of the PPE-induced COPD model
How we create the PPE-induced COPD model
For creating the PPE-induced COPD model we use female C57BL/6J mice that are 7-8 weeks old as a base and administer the elastase intratracheally using the Microsprayer®. After induction, the standard treatment period is 21 days before sacrifice. However, the treatment period as well as the treatment frequency and administration route of your compound are customizable.
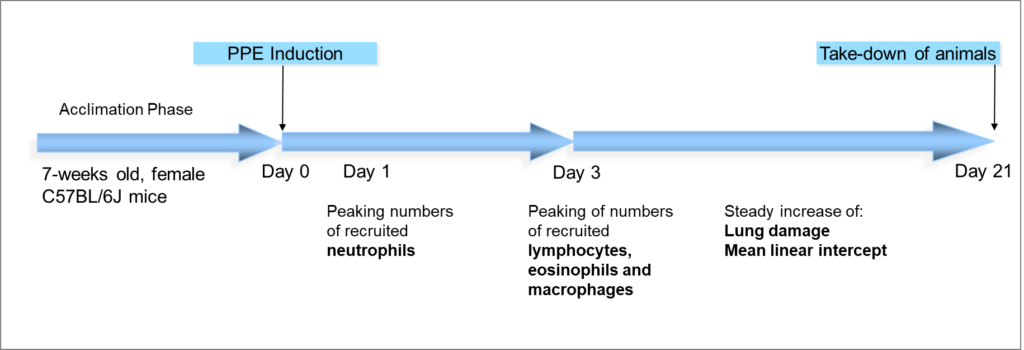
Fig. 3: COPD development in the PPE-induced pulmonary emphysema model
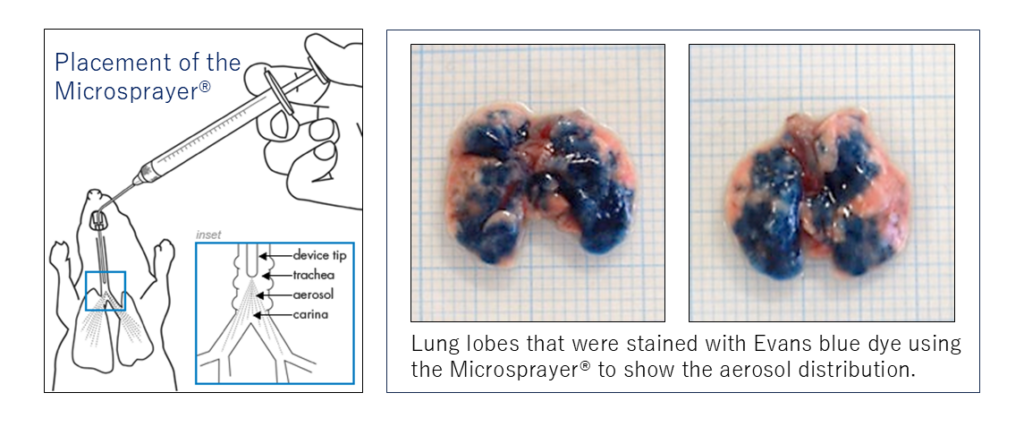
Benefits of using the Microsprayer® for COPD induction
The Microsprayer® is an application device for the intratracheal administration of aerosolized compounds such as PPE for the induction of COPD pathology in model mice. By administering the elastase through this route, we can create a more uniform phenotype in comparison to other routes. This is an especially beneficial feature of our model to facilitate the reproducibility of robustness of obtained data.
Analysis Items & Key Endpoints
Please find below the list of analyses that we can perform on samples from the PPE-induced COPD model.
|
BALF and lung analysis |
|
| BALF
Total cell counts Clee differentials (monocyte, lymphocytes, neutrophil, eosinophil) ELISA
Lung analysis ELISA
|
|
|
Histopathological analysis |
|
| HE staining (mean linear intercept)
Other IHC stainings
|
|
|
Gene Expression analysis |
|
| TNFa
MMP-9 etc.
|
|
|
Lung function markers |
|
| SpO2 (Saturation Pulse Oxygen)
FVC (Forced Vital Capacity)
|
|
|
Lung tissue density analysis |
|
| MicroCT analysis | |
Note: With the progression of the disease, the lung lobes become increasingly fragile so that the collection of BALF and samples for histological analysis or gene expression analysis from the same mouse is not possible. In case you want to include both types of analyses in your study, we usually suggest to simply increase the number of animals and subdivide each treatment group by analysis item to be performed.
In case you are more interested in pulmonary fibrosis models, please also check out our “Bleomycin-induced IPF model”.
Contact us
If you would like to discuss this model or have any open questions, we would be grateful, if you would reach out to us via the contact button below and we will be happy to support you.
