SMC MODELS
Bleomycin-induced pulmonary fibrosis model
The Bleomycin-induced pulmonary fibrosis model
The Bleomycin-induced pulmonary fibrosis model (IPF) is one of the most widely used models for preclinical pharmacological drug evaluations for new drug candidates indicated for IPF treatment. However, there is a vast number of different Bleomycin (BLM) models to choose from that vary in the concentration of administered BLM, the administration route and frequency as well as the in-life part of the preclinical study.
In the following, you will find the introduction and key parameters of the Bleomycin-induced IPF models that we have established, optimized and validated at SMC Laboratories.
The disease background: Interstitial Pulmonary Fibrosis (IPF)
Interstitial Pulmonary Fibrosis (IPF) is a chronic lung disease of yet unknow origin and without cure, in which alveolar epithelial cells are damaged and the lung tissue successively develops an increasing amount of fibrotic tissue. Over time, those lungs lose their ability for O2/CO2 gas exchange so that the patient needs a lung transplant. So far, there is no cure available for this disease and also the approved treatments for this disease are very limited in number and treatment success. Therefore, the need for new and improved treatment options is very large.
How do we create the Bleomycin-induced pulmonary fibrosis model?
We create our BLM-induced IPF model by administering the Bleomycin directly into the trachea of the anesthetized C57BL6J mice using a device called the Microsprayer® (explained below). Furthermore, our BLM model has been optimized for a balance between developing sufficient fibrosis severity and a tolerable survival rate.
Single-dose Bleomycin-induced pulmonary fibrosis model
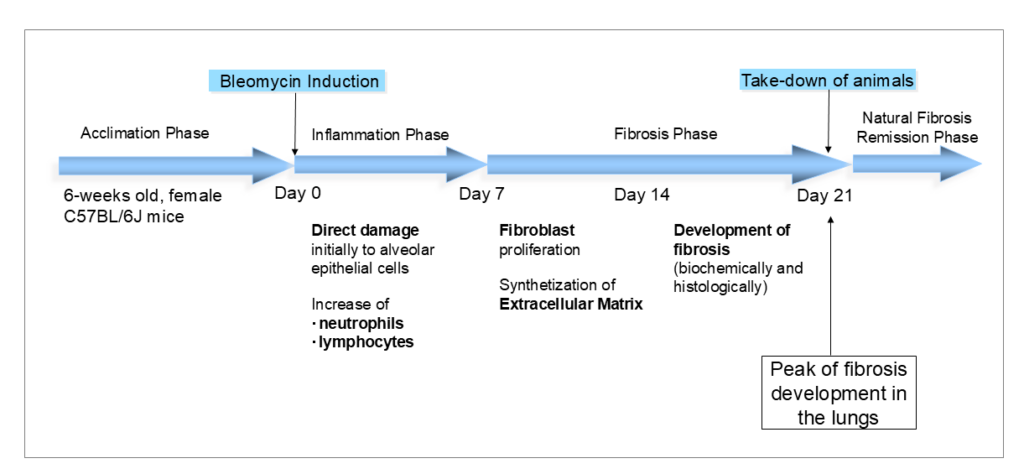
The single-dose Bleomycin-induced IPF model is created my administering one dose of 3mg/kg intratracheally using the Microsprayer®. Using this administration route results in a fibrosis development focused very locally in the lung tissue, but if you are looking for a more systemic fibrosis model, please also check our “Bleomycin-induced SSc-ILD model”.
The intratracheal administration of Bleomycin induces the inflammation phase which shows direct damage to the alveolar epithelial cells, an influx of immune cells and an increased release of inflammatory cytokines. From about day 7, the fibrosis phase starts. During this phase, we see the proliferation of fibroblasts and an increase in fibrotic tissue over time. The fibrosis severity in this model peaks at day 21, before the fibrosis goes into remission naturally.
Multiple-dose Bleomycin-induced pulmonary fibrosis model
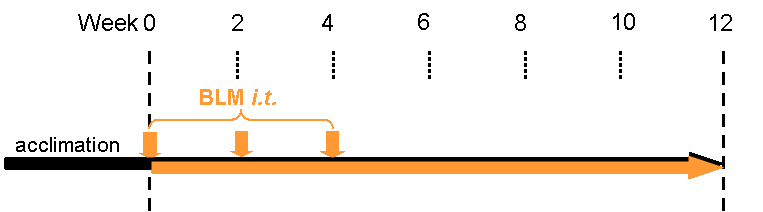
In the multiple-dose Bleomycin-induced IPF model, we administer the BLM at 3 time points (day 0, day 14 and day 28). By this administration pattern, we can extend the fibrosis development from 3 weeks (single-dose) to 12 weeks (multiple-dose) and hence increase the potential treatment period for your drug candidate.
Benefits of using the Microsprayer® for IPF induction
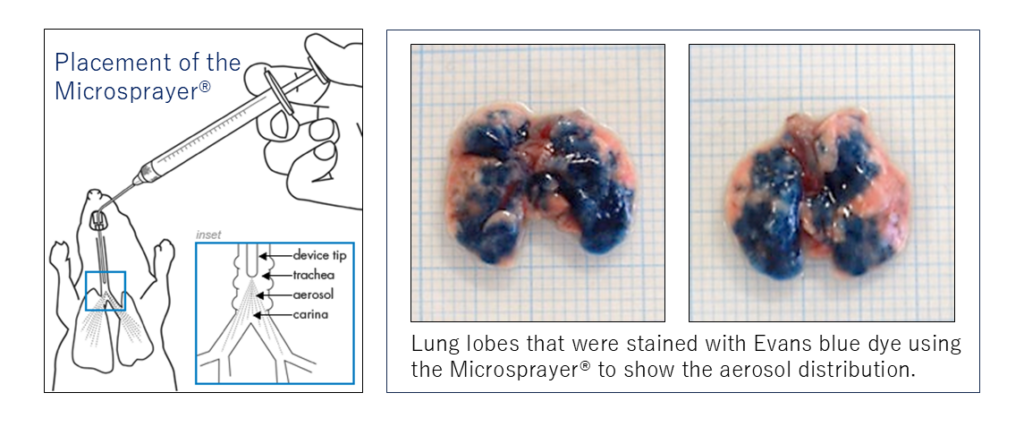
The Microsprayer® is a device that is inserted into the trachea of the mice and produces an aerosol of the Bleomycin. By using this administration route, we see less variability in the fibrosis development throughout the lung lobes in the individual as well as between the mice within one study. Even across different studies, this model shows a robust phenotype, which makes it easier to reproduce previous data. The biggest benefit of this model is that it can provide a more uniform phenotype in comparison to other administration routes.
Sampling scheme, Analysis Items & Key Endpoints
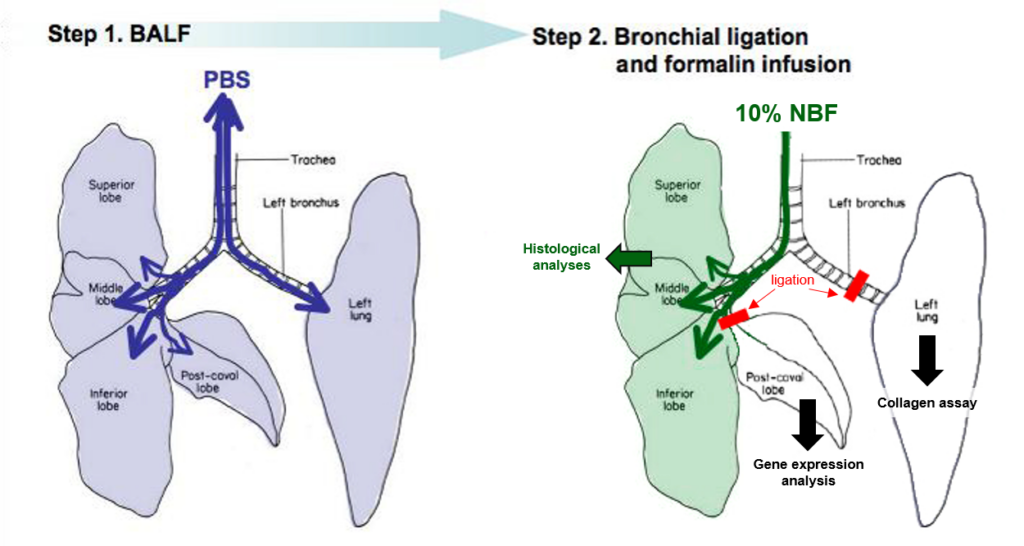
For obtaining the different lung samples in this model, we first collect the BALF and afterwards ligate this the left lung and post-caval lobe, which will be used in the analysis for collagen assay and gene expression, respectively. The other lobes are fixed with 10% NBF for histological analysis.
|
BALF and lung analysis |
|
| BALF
Total cell counts Clee differentials (monocyte, lymphocytes, neutrophil, eosinophil) ELISAs (TIMP-1, TGF-b etc.)
|
Lung analysis
Hydroxyproline (collagen) ELISAs (TIMP-1, TGF-b etc.)
|
|
Histopathological analysis |
|
| Evaluation of fibrosis
Sirius-red staining (fibrosis area) Masson trichrome staining (Ashcroft score, modified Ashcroft score) IHC for a-SMA (quantification)
|
Evaluation of inflammation
HE staining (lung injury score) IHC for inflammation markers |
|
Gene Expression |
|
| Fibrosis-related genes
TIMP-1 a-SMA CTGF Col1 Col3 etc.
|
Inflammation-related genes
MCP-1 IL-6 FLAP etc. |
|
Lung function markers |
|
| SpO2 (Saturation Pulse Oxygen)
Plasma MMP7FVC (Forced Vital Capacity)
|
|
|
Lung tissue density analysis |
|
| MicroCT analysis | |
Contact us
If you have any questions about the Bleomycin-induced IPF model or if you would like to have a free consultation for your drug development project, f. ex. regarding the best study design or our well-tested positive controls, please send us an inquiry through the contact button below.
We are happy to support you!
