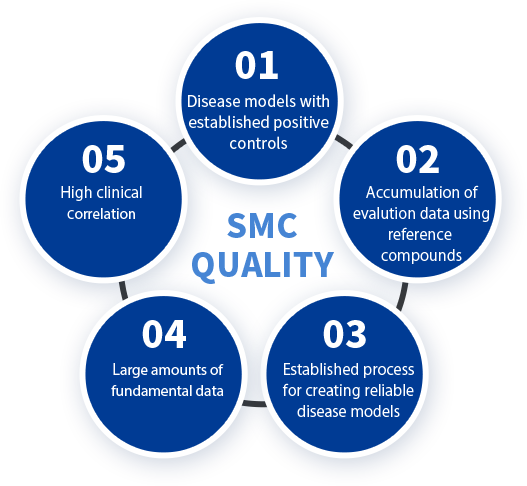
SMC QUALITY
We provide our clients with pharmacology studies that meet SMC quality and data that can be directly correlated to the results of clinical trials.
SMC QUALITY
SMC quality consists of the following five items.
- 1Setting positive control drugs for each disease model
- 2Accumulation of evaluation data using reference compounds
- 3Establishment of reproducible model creation technology
- 4Accumulation of abundant basic data
- 5Model suitability (linking the model to clinical conditions)
Model suitability (linking the model to clinical conditions)
CRO Consultation
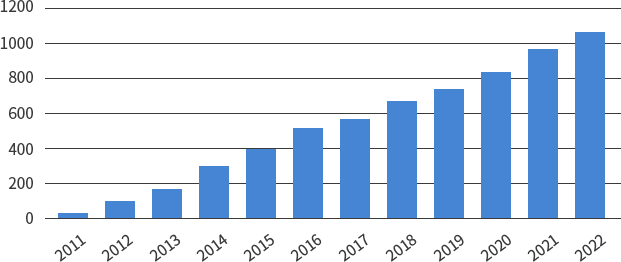
SMC Laboratories have supported over 1,000 clients worldwide.
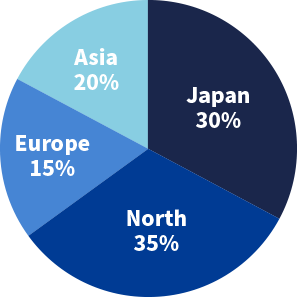
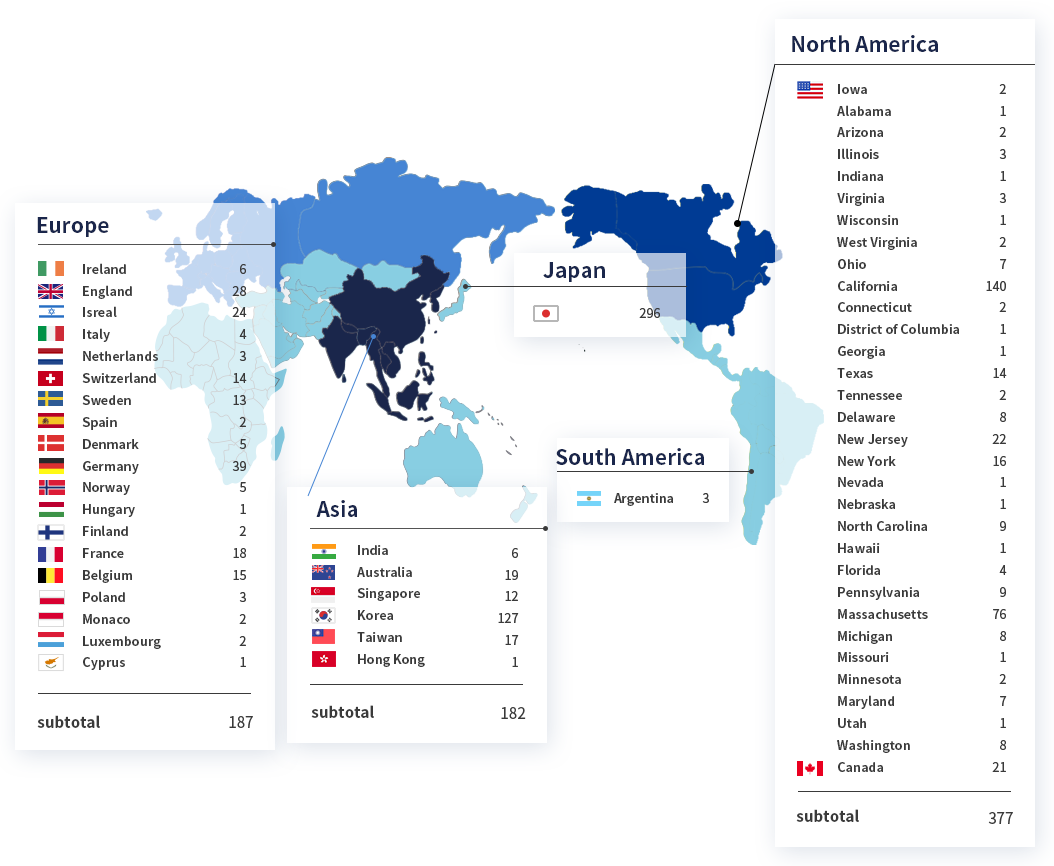
Number of clients
Region
Setting positive control drugs for STAM™/IPF model
Track record of evaluating a wide variety of test substances
- Long history of pharmacology study and evaluation of various modality of test substances such as small molecular compounds, antibodies, nucleic acids, cells, etc.
- Guaranteed integrity of the pharmacology study by setting Telmisartan as a positive control in STAM™ mice study.
In addition, we have experience in evaluating compounds such as OCA and CVC, which are currently undergoing clinical trials, and can be incorporated into the pharmacology study as target drugs. - Our clients have published test results using STAM™ mice in papers and at international conferences, with over 70 papers and 90 conferences to date.
In addition, there are more than 15 overseas companies that are currently conducting clinical trials. They have used the results of pharmacology studies performed by our company in CTA applications. - In pharmacology studies using the IPF model, we guarantee the integrity of our pharmacology studies by setting Dexamethasone in prevention studies and Nintedanib in therapeutic studies as our positive control drugs to ensure the pharmacology study.
Abundant specialized knowledge regarding liver research
- We have accumulated cutting-edge industry information and know-how through discussions with our clients who are top runners in the development of MASH/NASH therapeutics.
- We offer 10 different liver disease models, including MASH/NASH and liver fibrosis models, and have accumulated data for each model.
- We participate in international conferences such as NASH Summit, AASLD, and DDW, gaining cutting-edge knowledge in the field of liver research.
CRO specialized in MASH/NASH-HCC
- Based on the know-how we have accumulated, we have a system that allows us to propose and implement various studies according to client’s needs.
- Using our know-how in evaluating MASH/NASH and fibrosis, we are now able to conduct third-party evaluations of pathological specimens of MASH/NASH and fibrosis models created by clients.
We can evaluate, analyze, and propose fibrosis based extensive experience.
- Based on our extensive experience in chemical staining and immunostaining related to fibrosis, we have a capability of evaluating fibrosis or establishing new study designs tailored to your needs.
PATENTS
International Patent Number: WO2011/013247 Title of the invention: "Steatohepatitis-Liver Cancer Model Animal"
Patent number: 2009-178143 Title of invention: Steatohepatitis-liver cancer model animal
Case study
-
Galectin Therapeutics, Inc. (NASDAQ: GALT) conducted a pharmacology study targeting fibrosis using STAM™ mice.
A phase 2b trial was conducted in NASH cirrhosis.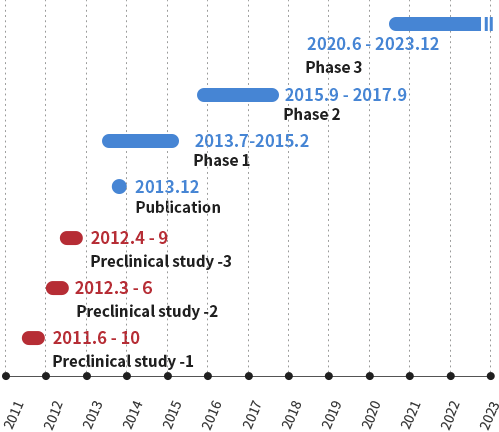
Our area of responsibility

-
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE:CFBI) conducted a pharmacology study targeting NASH using the STAM™ model. Phase 2 trials targeting liver fibrosis stage NASH are underway.
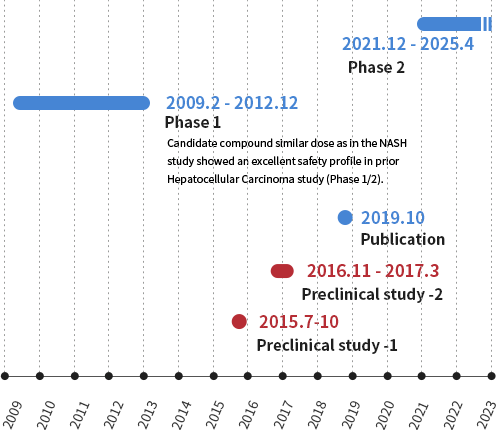
Our area of responsibility

